Carboxymethyl cellulose
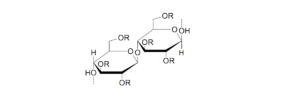
Cellulose is a natural polymer made of D-glucose units linked by
(1,4)-β-glucoside bonds. Cellulose is a natural, basic construction component of plants. It’s molecular weight has a significant impact on it’s and it’s derivative’s
physical characteristics , being dependent on the material source and the way of processing. Cellulose is most commonly used i paper industry, but also to produce synthetic fibres and explosives . Industrially the most important cellulose derivatives are i.e. ethers of cellulose (fig. 1):
chemical formula
The prevailing majority of cellulose ethers are soluble in water and
some of organic solvents.
Cellulose ethers are cellulose derivatives, in which the hydroxyl groups are partialy or entirely replaced by ether substituents. Every β-D-anhydroglucose unit,
repeated in cellulose, is accessible for ether groups in C-2,
C-3, C-6 positions. Ether substituents are in general low molecular alcoxy groups
including from 1 to 4 carbon atoms, which can be substituted by further
functional groups, like carboxylic group, hydroxyl or amino group.
The degree of estrification is different for each substituent as DS (degree of subsitution). DStakes a value from 0 to 3 and is equivalent to
an average hydroxyl estrification in a anhydroglucose unit, i.e. if such a unit has one of hydroxylic groups substituted by an ether group,
it’s DS. will be 0,5;if two groups are substituted, DS. will by 1 etc.
Cellulose etherification is conducted in an alkaline environment, usually using
sodium hydroxide. Cellulose is first treated with NaOH giving alkalicellulose,
which reacts with the etherifying reagent. One of the mainly used ether production method is the
Williamson reaction (chart 1).
The industry most commonly uses methyl chloride, ethyl chloride and sodium chloroacetate as RX etherifying agents. The reaction uses a stechiometric amount of sodium hydroxide.
Other etherification methods are the addition reactions of cellulose to:
- epoxy group, catalysed with acid, in which R=H, Me or Et,
- active double bond in alkaline environment (in this reaction, the double carbon bond has a substituent which highly attracts electrons, like CN, CONH2 or SO3Na groups).
The stages of industrial cellulose ether production:
- Grinding
- Activation (alkalinization) with NaOH water solution
- Heterogenous etherifying agent reaction
- Neutralisation
- Cellulose raw ether isolation
- Salt and side product purification
- Drying
- Conditioning
Activation – grinded cellulose is left to soak in an 18% to 30% rsodium hydroxide solution, which after a few hours results in cellulose maturation – a process of sodium cellulosane emergence and a polymer chain reduction i.e. under the action of oxygen present between the cellulose fibres.
In modern technology, a 30-70% NaOH solution is sprayed directly onto the rotary pipe, on powdered cellulose. Alternatively, the cellulose powder can be suspended
in an organic solvent, and a sodium hydroxide added afterwards.
In case of the Williamson etherification method, the amount of hydroxide
depends on the product’s DS value. but it shouldn’t be more than 1,5 mole
of the redundant NaOHin an anhydroglucose unit, for it lowers the reaction efficiency
due to substrate hydrolisys.
Etherification and neutralisation – in case of low molecular alkyl chlorides or epoxydes as etherifying agents, the reaction is conducted in autoclaves
made from acid-resistant steel under the pressure of 3 MPa in an increased temperature (50-100oC). The time of the reaction ranges form 0,5 to 116 h. After the reaction is completed, the reactor content
is cooled down and neutralised with acid (usually hydrochloric, nitric or acetic).
Secretion – ethers soluble in organic solvents or thermal coagulating
are cleared with the use of hot water. The majority of
hydroxyalkyl or carboxyalkyl derivatives is hotwater-soluble. The salts are then removed from the raw product periodically or constantly.
The clear product is secreted by filtering or centrifugation. To improve the properties of the final product, it is common to add a cross-linking agent.
Carboxymethyl cellulose
The carboxymethyl cellulose (CMC) production is simplier than the other cellulose ethers synthesis because:
- all the reagents are solid or liquid,
- all the production stages can be conducted at the atmospheric pressure,
- etherifying reagents – chloroacetate acid or sodium chloroacetate – easily available,
- etherifying reagents are very effective.
Due to the abovementiond, and also good thickening, water-stopping and colloid-protective properties, carboxymetyl cellulose is widely used
in the industry. Large amounts of CMC are used to produce laundry detergents, cosmetics and in the paper industry. A highly clear CMC is also used in the food industry.